The Kenya Poisons and Pharmacy Board has recalled Zantac, a heartburn relief medication, following findings that linked it to cancer.
Zantac, which is also packaged as Neotack, was found to have carcinogenic traits in its main component - Ranitidine - by the Food and Drug Administration (FDA) in the United States.
"In order to safeguard the health of Kenyans, you are instructed to carry out a level two recall of all ranitidine products from the Kenyan market.
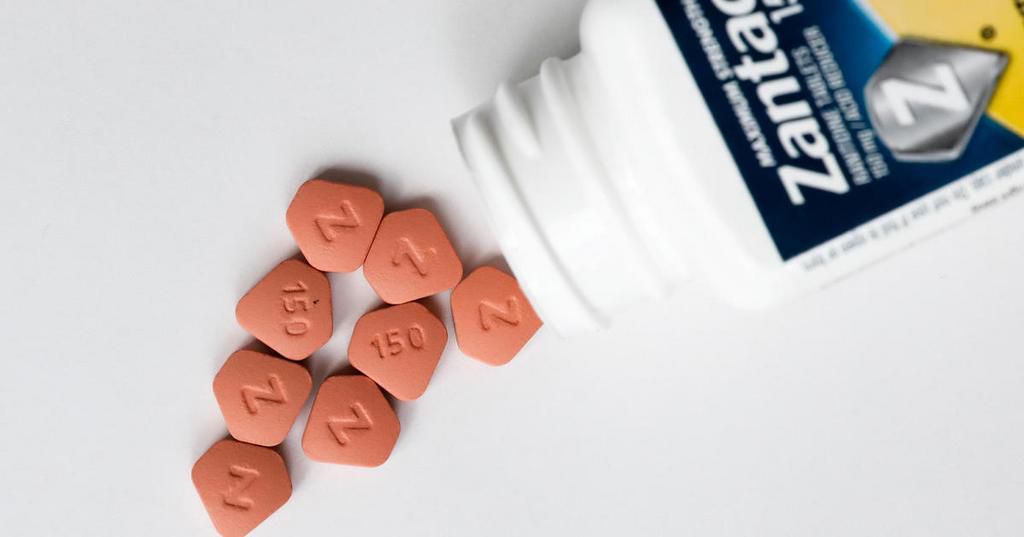
“In addition, you are required to submit to the board the details of all the products that you have imported into the country in the last three years," a circular signed by PPB CEO Dr Fred Siyoi read in part.
The US FDA department has been conducting tests on samples of heartburn, blood pressure and heart medicines specifically for a component, N-nitrosodimethylamine (NDMA) which can cause cancer.
Augmentin antibiotic
In August, PPB alerted Kenyans on fake Augmentin tablets that had infiltrated the Kenyan market and clarified on how to tell if the antibiotic given is genuine.
The fake antibiotics were being circulated during "flu season", it is one of the main antibiotics prescribed for respiratory tract infections.
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)